 Science Matters
Science Matters
““To see a world in a grain of sand, or heaven in a wildflower.
Hold infinity in the palm of your hand, and eternity in an hour."
~ William Blake
In this series Dr. Gordon Maclean has described the scientific principles underlying climate change. In this piece he explains greenhouse gasses. How does this relate to the light spectrum? Read on!
Greenhouse Gasses: How They Work
By Gordon Maclean, Ph.D.
Most people are familiar with the terms “greenhouse effect” and “greenhouse gasses.” My previous column dealt with whether reforestation is a possible way to remove enough greenhouse gasses from the air to reverse the effects of greenhouse gasses. Carbon dioxide (CO2) and methane (CH4) are the most significant greenhouse gasses in our atmosphere. But how exactly do greenhouse gasses work?
As an analogy, the term “greenhouse effect” is sort of good. A greenhouse is a structure made of glass or plastic that allows the sun inside. Objects are warmed by the sun, which warms the air around them, but the glass or plastic creates a physical barrier that traps the warm air and prevents it from dissipating.
Greenhouse gasses do not present a physical barrier like glass or plastic (which is why I used “sort of good” above.) Being mixed across the atmosphere, they work as a non-physical barrier.
To understand how they work, we need to understand the nature of the electromagnetic (EM) spectrum, of which light that we can see is a very, very small portion. The EM spectrum covers everything from gamma rays to radio, with a LOT of different categories in between, including visible light and infrared.
All parts of the EM spectrum interact with objects in three ways, they can be absorbed, reflected, or transmitted (passes through.)
The figure below is a simple outline of the EM spectrum, taken from this site (https://imagine.gsfc.nasa.gov/science/toolbox/emspectrum1.html.) If you would like further information on the EM spectrum, I would highly recommend this as a starting point.
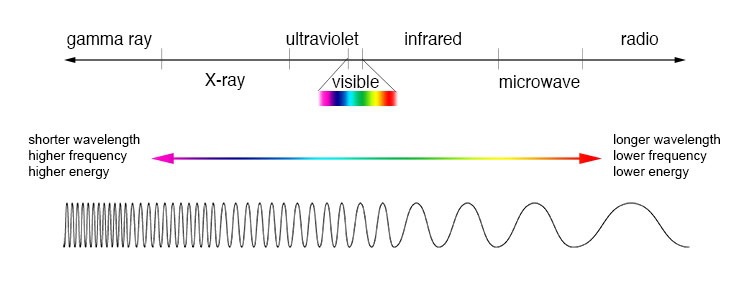
When we see colors, we see the reflected portion of the visible EM. Shadows are cast when there is little or no transmittance of the visible EM. And the difference is the EM that is absorbed by the object. An asphalt road gets so hot on a sunny day because it is absorbing most of the sun’s light and reflecting very little (which is why it appears black to our eyes.)
There are two components to infrared “light.” The first is just beyond what our eyes can see, and we can make cameras that are sensitive to infrared light (in the old days, we could do the same with film.) We call this “near-infrared.” Near-infrared emitted EM (not reflected, trees, people, houses will reflect the sun’s EM) represents temperatures just below what we could see with our eyes; think of an electric stove heating element, just before it glows red.
The second type is “far-infrared.” All objects give off (emit) far-infrared when they reach a certain temperature. Far-infrared objects emit heat at temperatures we are accustomed to in our day-to-day lives. We need a particular type of device to measure or record this emitted infrared energy. Thermal scanners are specifically designed to perform this task. An example of using a thermal scanner to detect emitted heat would be looking for people who have a fever (from https://www.nbcnews.com/id/wbna30523865.)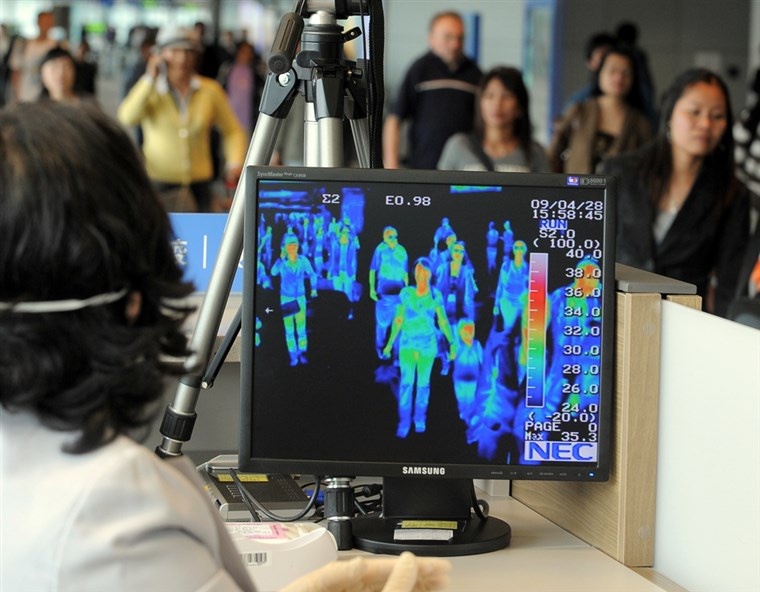
So, we have the sun coming through our atmosphere and warming up everything during the daytime, converting visible light energy into far-infrared (heat) energy, and that thermal energy is immediately emitted back out, eventually into space. At night, without the sun, the far-infrared energy continues emitting even though there is no light coming in (that is why the sidewalk still feels warm at night.) When everything is in balance, we do not get too warm during the day, and we stay warm at night.
Here is where the greenhouse effect comes into play. Remember above that we said that all EM energy could be absorbed, reflected, or transmitted. Every object reacts with different parts of the EM spectrum differently; that is how the physician’s X-ray machine works and why we can receive radio signals inside a house.
Greenhouse gasses are transparent in the visible part of the spectrum, which is why we cannot see carbon dioxide or methane. However, these gasses are incredibly effective at absorbing most portions of far-infrared EM energy (heat.)
I wish I could show you an image of the Earth showing this effect. But no one has put a satellite in orbit capable of imaging these wavelengths of EM simply because they would show, literally, nothing useful. At best, our planet in these areas of the far-infrared spectrum would look similar to the planet Venus.
 Image from https://www.space.com/12437-venus-photos-planets-venusian-solar-system.html
Image from https://www.space.com/12437-venus-photos-planets-venusian-solar-system.html
There is, however, a small “window” where the atmosphere is transparent to emitted infrared. Weather satellites are designed to collect data from this “window” to see clouds during the night.
Essentially, greenhouse gasses work as a layer of transparent insulation, trapping more emitted far-infrared (heat) radiation as we add more of these gasses into the air. The more insulation we add, the less of this far-infrared radiation is allowed to escape into space.
For more information on how greenhouse gasses work, this is an excellent resource http://www.ces.fau.edu/nasa/module-2/how-greenhouse-effect-works.php.
So that is how greenhouse gasses work. The importance of the WHY cannot be understated.
We can get a pretty good idea of past CO2 levels from a variety of sources. The best source is from directly measuring the air trapped in small ice bubbles tens to hundreds of thousands of years ago in the ice in Greenland and Antarctica. These records, along with direct measurements of our air for the past 150 years, show that our CO2 levels are currently at the highest level they have been for at least the past 800,000 years. Using other methods to measure atmospheric CO2, there are indications that we are currently at the highest levels in about 20,000,000 years and still on an upward trajectory. If you are interested in more details on our planet’s CO2 history, I would recommend this source https://earth.org/data_visualization/a-brief-history-of-co2/.
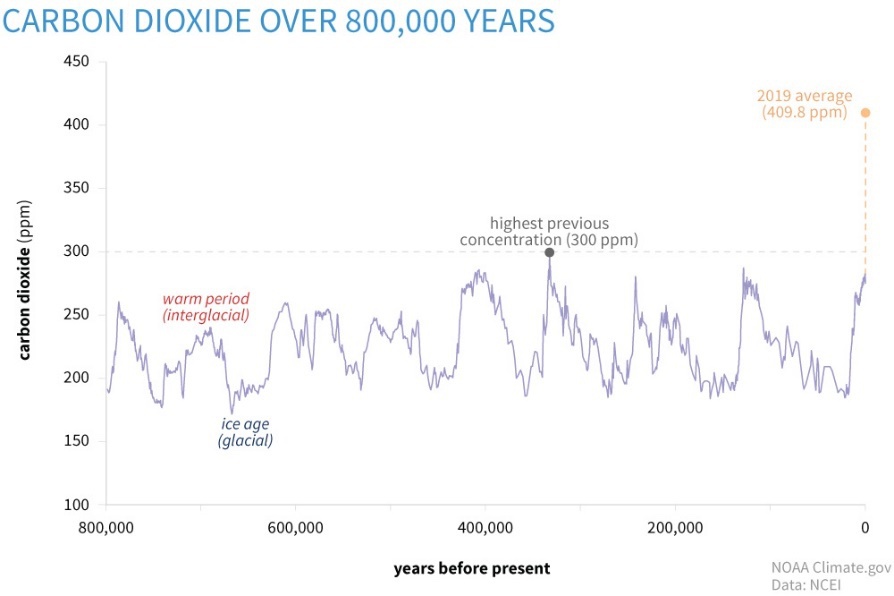 Image from https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide
Image from https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide
Previously...
- Science Matters: Angry Weather: Heat Waves, Floods, Storms, and the New Science of Climate Change
- Science Matters: An Immense World
- Science Matters: Fractals in Nature
- Science Matters: Fibonacci: Nature’s Mathematics
- Science Matters: Climate Change and Extreme Weather Events and You
- Science Matters: Raging Wildfires
- Science Matters: Wolf-Moose Population Studies
- Science Matters: Bird Vision
- Science Matters: Greenhouse Gasses: How They Work
- Science Matters: Reforestation and Climate Change
- Science Matters: The Carbon Cycle: Part Two
- Science Matters: The Carbon Cycle: Part One
